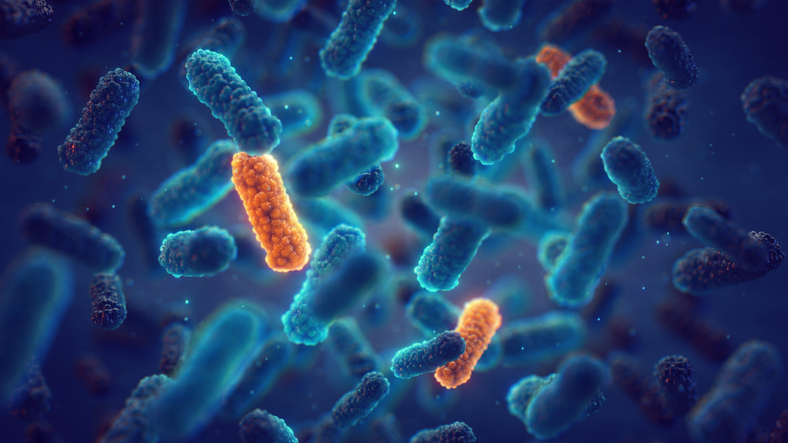
Antibiotic resistance is escalating into a serious global health crisis, with projections indicating over 10 million deaths annually by 2050. This rise in antibiotic-resistant bacteria is particularly evident in hospital environments, sewage treatment facilities, and animal farming operations. In a significant breakthrough, researchers from the University of California, San Diego (UCSD) have developed a new CRISPR-based technology aimed at eradicating antibiotic-resistant genetic elements from bacterial populations.
A study titled “A conjugal gene drive-like system efficiently suppresses antibiotic resistance in a bacterial population,” published in npj Antimicrobials and Resistance, introduces a tool called pPro-MobV. This second-generation technology builds on the concept of gene drives, which have been utilized to disrupt harmful traits in insect populations, such as those spreading malaria.
Ethan Bier, PhD, a distinguished professor in the department of cell and developmental biology at UCSD and the study’s corresponding author, explained, “With pPro-MobV, we have brought gene-drive thinking from insects to bacteria as a population engineering tool.” This innovative technology enables researchers to take a small number of bacterial cells and allow them to neutralize antibiotic resistance across a larger population.
The origin of this technology traces back to a collaboration in 2019 between Bier’s lab and Victor Nizet, MD, a distinguished professor at UCSD School of Medicine. They first explored the Pro-Active Genetics concept, whereby a genetic cassette is introduced between bacterial genomes to inactivate antibiotic-resistant features. This cassette effectively targets antibiotic-resistant genes, restoring the bacteria’s sensitivity to standard antibiotic treatments.
The latest research has advanced this concept by developing a system that spreads the CRISPR cassette components through conjugal transfer. This new method capitalizes on natural bacterial mating tunnels, allowing the disabling elements to disseminate effectively. The researchers demonstrated this process within bacterial biofilms—structured communities of microorganisms that pose significant challenges in medical and environmental contexts due to their protective layers.
Biofilms are implicated in a majority of serious infections, making them particularly difficult to treat with conventional antibiotics. The potential applications of this technology span healthcare settings, environmental remediation, and microbiome engineering. Bier emphasized the importance of addressing biofilms in the fight against antibiotic resistance, as they represent one of the most formidable forms of bacterial growth encountered both in clinical environments and enclosed settings, such as aquaculture ponds and sewage treatment plants.
Interestingly, the study also revealed that components of the active genetic system could be delivered by bacteriophages—viruses that specifically target bacteria. Researchers envision deploying pPro-MobV in tandem with engineered phage viruses to enhance its effectiveness. Additionally, this genetic platform includes a safety feature that allows for the homology-based deletion of the gene cassette if needed.
“This technology is one of the few ways that I’m aware of that can actively reverse the spread of antibiotic-resistant genes, rather than just slowing or coping with their spread,” stated Justin Meyer, PhD, a professor in the department of ecology, behavior, and evolution at UCSD and co-author of the study.
The implications of this research are profound, offering hope in the battle against antibiotic resistance. As the world grapples with this escalating crisis, innovations like the pPro-MobV tool could play a pivotal role in safeguarding public health and ensuring effective treatments for bacterial infections.