Lithium-metal batteries, recognized for their potential in energy storage due to their high energy density and lightweight design, face a significant challenge from the growth of dendrites. These tiny, needle-like structures made of lithium can develop within the battery, leading to dangerous short circuits. Recent research from the Technical University of Munich (TUM) has revealed surprising insights about dendrite formation that could influence future battery safety and efficiency.
Fabian Apfelbeck, a physicist pursuing his doctorate under Prof. Peter Müller-Buschbaum, highlights that dendrites traditionally were believed to form only at the interface between the electrode and electrolyte. His study, published in Nature Communications, indicates that dendrites can also grow within the polymer-based electrolyte itself, challenging existing assumptions in battery research.
Understanding the Role of Electrolytes
Electrolytes play a crucial role in lithium batteries by facilitating the movement of lithium ions between electrodes, which is essential for current flow. The shift toward polymer-based electrolytes has been motivated by their stability and safety advantages over liquid electrolytes. Unlike liquids, polymers cannot leak or ignite, providing a layer of protection against short circuits. Apfelbeck notes, “Our measurements show that dendrite growth can also occur directly inside the polymer electrolyte—right in the material that is actually supposed to protect against dendrites.”
This unexpected finding sheds light on the internal dynamics of battery materials and the limitations of current protective measures. Prof. Müller-Buschbaum expressed surprise at the discovery, stating, “The fact that it also appears far away from that interface surprised us.” This insight opens new avenues for developing materials that can prevent internal crystallization processes, ultimately leading to safer and more durable energy storage solutions.
Innovative Techniques for Battery Research
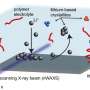
To conduct this research, the team employed a novel technique known as nanofocus wide-angle X-ray scattering. This method, executed at the German Electron Synchrotron (DESY) in Hamburg, allowed researchers to observe microscopic changes within the polymer electrolyte during battery operation for the first time. By utilizing an X-ray beam with a diameter of just 350 nanometers, the researchers could visualize the intricate processes occurring within the battery.
The study underscores the importance of continual innovation in battery technology, as the findings will aid in the design of more effective materials that mitigate the risks associated with dendrite formation. As the demand for safer and more efficient energy storage solutions grows, this research stands at the forefront of advancements in lithium battery technology.
In conclusion, the work of Fabian Apfelbeck and his colleagues at TUM not only challenges established beliefs regarding dendrite growth in lithium batteries but also paves the way for the development of improved battery technologies. As the global energy landscape continues to evolve, understanding these fundamental issues will be essential for enhancing the safety and performance of energy storage systems.