Researchers at the Weizmann Institute of Science have revealed a breakthrough in enzyme design that achieves catalytic efficiencies exceeding 100,000 M −1 s −1. This innovative work promises to create artificial catalysts that rival the performance of natural enzymes without extensive laboratory refinement. The findings were published in the journal Nature on July 14, 2025.
Natural enzymes are renowned for their ability to perform biochemical transformations rapidly and accurately. However, artificial catalysts have historically struggled to match their speed and efficiency. This new study, titled “Complete computational design of high-efficiency Kemp elimination enzymes,” demonstrates a fully computational approach to enzyme creation, setting a new standard for artificial enzymes.
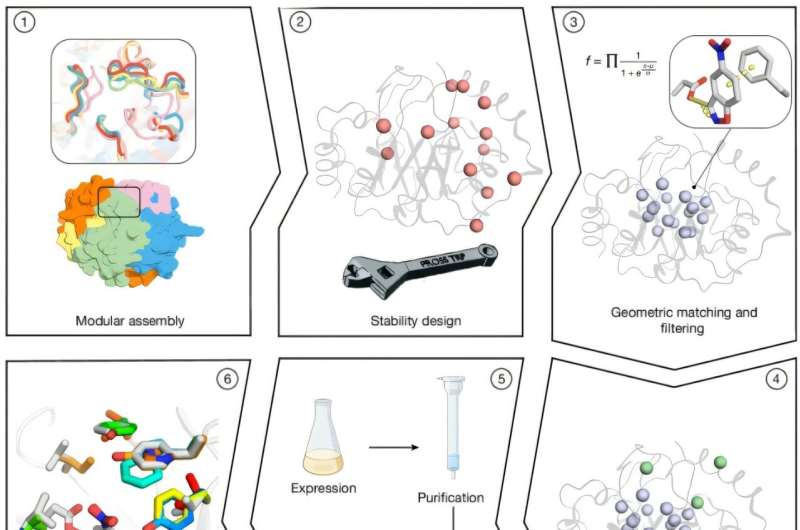
The research team embarked on this ambitious project by generating thousands of TIM-barrel backbones, which are one of the most common protein structures found in enzymes. Through a combinatorial assembly and design process, they combined fragments from homologous proteins. This structural design allows for optimal placement of catalytic and substrate-binding groups, significantly enhancing the enzymes’ performance.
To refine their designs, researchers utilized Rosetta atomistic calculations, resulting in millions of potential enzyme configurations. These were filtered based on an objective function that balanced energy and desolvation of the catalytic base. Ultimately, 73 designs were selected for experimental testing. Of these, 66 successfully expressed in soluble forms, and 14 exhibited cooperative thermal denaturation behavior, a sign of stability.
The introduction of 5 to 8 active-site mutations per variant led to impressive increases in catalytic efficiencies. Notably, one variant derived from Des61 achieved a kcat/KM of 3,600 M −1 s −1 and a turnover rate of 0.85 s −1. Even more remarkable, designs based on Des27 demonstrated catalytic rates that were 10–70 times higher than the original, with Des27.7 reaching a kcat/KM of 12,700 M −1 s −1 and a kcat of 2.85 s −1. A single-point mutation, which replaced Phe113 with leucine, propelled catalytic efficiency to an astounding 123,000 M −1 s −1 and a kcat of 30 s −1.
The researchers concluded that the combination of active-site preorganization and the ability to adopt multiple catalytically competent conformations distinguishes their designs from previous efforts. One of the best variants exhibited high stability, remaining effective at temperatures exceeding 85 °C, with catalytic efficiencies on par with natural enzymes.
These findings indicate that current atomistic computational methods are sufficiently reliable for generating efficient enzymes that maintain natural folds, eliminating the need for iterative laboratory screening or reliance on artificial intelligence-generated scaffolds. Looking ahead, advancements in modeling theoretical enzymes may pave the way for fully programmable biocatalysis, expanding the potential for applications in product manufacturing, therapeutic development, pollution remediation, and green chemistry.
The implications of this research could be broad, offering novel solutions for various industries and contributing to environmental sustainability initiatives. As the scientific community continues to explore these advancements, the future of enzyme engineering looks promising.
For further details, refer to the original research by Dina Listov et al in Nature (DOI: 10.1038/s41586-025-09136-2) and Zhuofan Shen et al (DOI: 10.1038/d41586-025-02054-3).