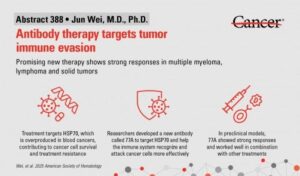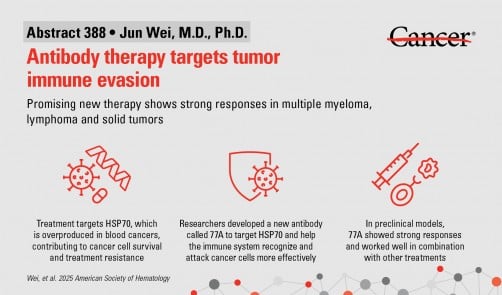
A groundbreaking preclinical study presented by researchers from The University of Texas MD Anderson Cancer Center has unveiled a new investigational therapy known as 77A. This antibody has demonstrated the potential to enhance immune responses against blood cancers, including myeloma and lymphoma, as well as various solid tumors. The findings were revealed at the 67th American Society of Hematology (ASH) Annual Meeting in Orlando on December 6, 2025.
Led by Jun Wei, M.D., Ph.D., an assistant professor of Lymphoma & Myeloma, and principal investigator Robert Z. Orlowski, M.D., Ph.D., the study highlights how 77A works by targeting a cancer survival protein known as HSP70. This protein is often overproduced in various cancers, allowing tumors to evade the immune system. By converting HSP70 into an immune system trigger, 77A activates both T cells and natural killer (NK) cells, reshaping the tumor environment and promoting lasting immune responses.
Mechanism of Action and Treatment Combinations
In laboratory models, 77A has shown to significantly enhance the effectiveness of existing treatments such as chemotherapy, radiation therapy, and immunotherapies. The antibody not only boosts the immune response but also improves the ability of immune cells to identify and destroy cancer cells. This combination therapy approach holds promise for patients, as it has demonstrated strong antitumor effects across multiple tumor types.
Moreover, initial tests with human immune cells indicate that 77A can enhance immune responses even in healthy donors, suggesting its versatility as a potential therapeutic option. The study’s authors believe that 77A could work synergistically with other advanced treatments, including adoptive T cell therapy, where patients receive laboratory-grown immune cells specifically designed to target their cancer.
Future Directions for Clinical Research
The encouraging results from this study pave the way for future clinical trials. Robert Z. Orlowski expressed optimism about the potential of 77A, stating, “These results give us confidence that 77A could become a versatile immunotherapy.” The next steps involve developing a humanized version of the antibody, which is anticipated to move into clinical trials aimed at evaluating its effectiveness in patients with various cancer types.
This research was supported by Blood Cancer United, the organization formerly known as the Leukemia & Lymphoma Society. A complete list of collaborating authors and their disclosures can be found alongside the study abstract.
The promise of 77A as a new therapeutic option marks a significant step forward in the battle against cancer, emphasizing the need for continued research and innovation in the field of immunotherapy.