A recent study led by Meizhen Wang at Zhejiang Gongshang University has uncovered promising insights into using plant-derived compounds to combat human bacterial pathogens (HBPs) in manure-amended soils. Published in the journal Biocontaminant on November 26, 2025, the research indicates that these natural extracts can significantly disrupt the communication systems used by harmful bacteria, potentially reducing the spread of antibiotic resistance and virulence.
The application of manure is crucial for maintaining soil fertility and enhancing crop yields. However, it can also introduce HBPs that may harbor antibiotic resistance genes (ARGs) and virulence factor genes (VFGs) into agricultural environments. These pathogens can move into crops and subsequently enter the food chain, raising substantial concerns for both ecosystems and human health. Traditional methods to mitigate these risks, such as biochar or engineered nanoparticles, often come with high costs or environmental drawbacks.
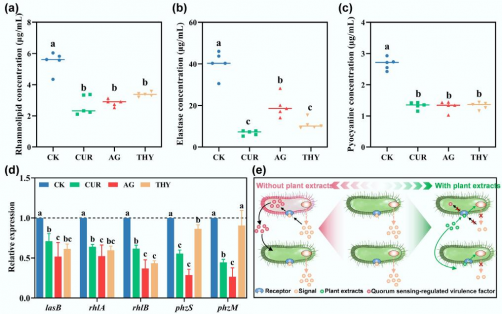
This study explored the effects of three plant-derived compounds: curcumin, andrographolide, and thymol. Using microcosms of manure-amended soil, researchers combined advanced techniques like metagenomic profiling and molecular docking analyses to investigate how these compounds affect HBPs and their associated risks. A comprehensive database identified a total of 323 HBPs, and the study carefully assessed how their abundance and community composition shifted after treatment with the plant extracts.
Results revealed that the application of these extracts led to a reduction of approximately 25% to 28% in total HBP abundance, with a marked suppression of pathogens associated with the Actinobacteria and Proteobacteria phyla. Additionally, there was a significant decline in key risk indicators, including a reduction of ARGs by 20% to 27%, VFGs by 6% to 11%, and mobile genetic elements (MGEs) by 25% to 34%. These findings highlight a strong correlation among these elements, underscoring the interconnectedness of pathogenicity and resistance traits.
The mechanisms behind these effects were also thoroughly investigated. The study found that plant extracts inhibited quorum sensing (QS)—the communication network that bacteria use to coordinate their responses—by decreasing both the abundance of QS genes and the levels of acyl-homoserine lactone signals. This disruption resulted in a notable downregulation of QS-regulated genes, leading to reduced secretion of virulence factors, a 40% decrease in biofilm formation, and suppression of gene transfer related to ARGs and VFGs by as much as 90%.
To further validate these findings, molecular docking studies demonstrated that the plant compounds bind to the QS receptor LasR with a higher affinity than native signal molecules. This competitive binding effectively blocks bacterial communication, revealing a non-bactericidal approach to mitigating soil-borne pathogen risks.
The implications of this research position plant extracts as an environmentally friendly alternative for improving soil health and safety in agricultural settings. Unlike conventional antibiotics or nanomaterials, these compounds disarm pathogens rather than eliminate them, thereby reducing the selective pressure that often leads to increased resistance.
With the escalating global challenge of antibiotic resistance, the potential of utilizing plant-derived solutions offers a fresh perspective on sustainable agricultural practices. This study paves the way for future research into natural compounds that can enhance soil safety and promote healthier ecosystems without compromising agricultural productivity.







