CERo Therapeutics Holdings, Inc., a cellular immunotherapy company based in South San Francisco, has announced the presentation of significant interim data from its Phase 1 clinical trial, known as CERTAIN-T, during the upcoming Tandem Meeting of the American Society of Transplantation and Cellular Therapy (ASTCT) and the Center for International Blood and Marrow Transplant Research (CIBMTR). The meeting takes place from February 4 to 6, 2026, in Salt Lake City, Utah.
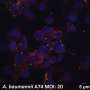
The highlighted data showcase the efficacy and safety profile of CERo’s lead compound, CER-1236. Preliminary results from the initial cohort reveal a positive safety and tolerability profile, along with a remarkable in vivo cell expansion, showing an approximate 20–70-fold increase that peaked between days 7 and 14. Following this peak, the expansion demonstrated prolonged persistence, indicating potential long-term benefits for patients.
Among the compelling outcomes shared in the presentation is the case of an index patient diagnosed with acute myeloid leukemia (AML), which progressed from myelodysplastic syndrome (MDS). This patient underwent a total of four infusions of CER-1236 over five months at the lowest dose level. Prior to the treatment, the patient experienced frequent platelet transfusions. However, post-treatment, he achieved transfusion independence lasting over two months, surpassing the commonly accepted durability benchmark of 56 days.
As CERo Therapeutics continues to explore novel phagocytic mechanisms and new therapeutic targets, the results from the CERTAIN-T trial underscore the potential of CER-1236 in treating patients with hematological malignancies. The company’s commitment to advancing cellular immunotherapy is evident, and the upcoming presentation at the ASTCT and CIBMTR meeting will provide an important platform for sharing these findings with the medical community.
Investors and stakeholders are keenly attentive to the developments surrounding CERo Therapeutics, particularly as the company navigates the complexities of bringing innovative therapies to market. The results presented during this critical meeting could influence future research directions and clinical applications, as well as shape investor sentiment regarding the company’s ongoing projects.
With a focus on safety, efficacy, and patient outcomes, CERo Therapeutics is poised to make a significant impact in the field of immunotherapy, potentially transforming the treatment landscape for patients with challenging conditions like AML and MDS.