Recent research reveals that cancer cells possess a remarkable ability to manipulate neighboring healthy cells by using their mitochondria, the energy-producing components of cells. This groundbreaking study highlights how tumors can not only steal mitochondria from surrounding cells but also transfer their own mitochondria to reprogram nearby healthy cells, effectively turning them into supportive allies for the cancer.
In findings published on August 28, 2023, in the journal Nature Cancer, a team led by Sabine Werner, a biochemist and cell biologist at ETH Zurich, discovered a crucial protein called MIRO2. This protein acts as a facilitator for mitochondria transfer from cancer cells to neighboring fibroblasts, which are connective tissue cells. “Without MIRO2, cancer cells can’t send their mitochondria to fibroblasts,” Werner stated, underscoring the protein’s significance in the reprogramming process.
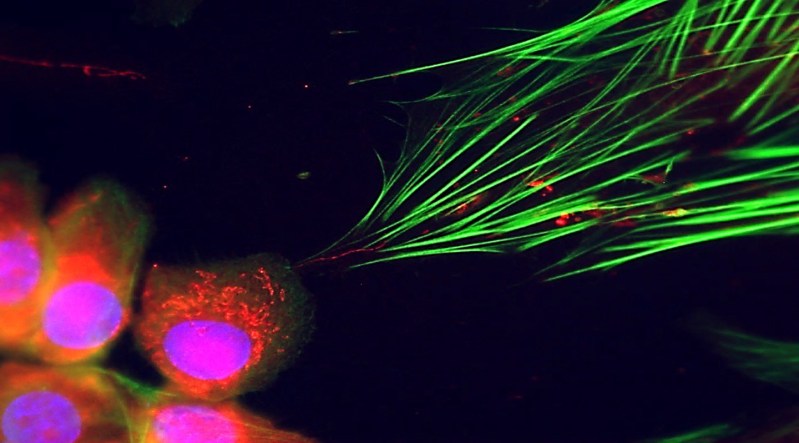
The research team originally focused on how cancer cells communicate with fibroblasts, but their observations led to an unexpected discovery. They noticed long, thin structures connecting the two cell types, resembling tunneling nanotubes, which are known for transporting mitochondria and other materials between cells. Intrigued by this finding, the researchers confirmed the presence of cancer cell mitochondria entering fibroblasts, a phenomenon previously unreported.
This influx of mitochondria resulted in the fibroblasts exhibiting accelerated growth and increased activity of genes associated with cancer, effectively transforming them into supportive agents for the tumor. When these modified fibroblasts were injected into mouse ears alongside cancer cells, they contributed to tumor formation, demonstrating their role as “turncoat” cells in the cancer microenvironment.
The implications of this study extend beyond fibroblasts. According to Yosuke Togashi, a molecular biologist at Okayama University in Japan, cancer cells can also transfer mitochondria to immune cells, potentially undermining their cancer-fighting abilities. Earlier research by Togashi’s team indicated that this mitochondrial transfer process could significantly influence the behavior of immune cells in the presence of tumors.
As the field of mitochondrial transfer research expands, experts like Jiří Neužil from the Czech Academy of Sciences recognize the significance of these findings. Neužil expressed confidence in the occurrence of mitochondrial shunting from cancer cells to fibroblasts and acknowledged the wave of new questions raised by Werner’s results. “What is the driving force behind it? What is the trigger? What is the mechanism?” he pondered.
Despite the early stages of this research, the potential for new therapeutic approaches is evident. Understanding the mechanisms behind mitochondrial transfer could lead to innovative strategies for combating cancer by disrupting the communication between tumors and their surrounding environment. Werner emphasized the importance of curiosity and exploration in scientific research, noting that following unusual leads can yield profound insights.
As this field continues to evolve, the revelations about cancer cells’ ability to manipulate their environment through mitochondrial transfer may pave the way for significant advancements in cancer treatment and management.






