Researchers have uncovered critical insights into how mitochondrial metabolism supports T-cell proliferation and prevents T-cell exhaustion, especially in the context of cancer and chronic infections. Led by Navdeep Chandel, Ph.D., at the Division of Pulmonary and Critical Care, this study was published in Nature Immunology and reveals significant implications for developing targeted immunotherapies.
The mitochondrial electron transport chain (ETC) plays a vital role in cellular energy production by facilitating electron transfer and generating ATP, the energy currency of cells. Previous research from Chandel’s laboratory established that mitochondrial ETC function is essential for the proliferation of CD8+ T-cells, which are pivotal in combating cancer and viral infections. However, the specific functions of the mitochondrial ETC that foster CD8+ T-cell responses and proliferation had remained elusive until now.
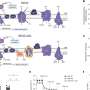
To further investigate these processes, the team developed mice that were deficient in mitochondrial complex III, one of the four complexes within the mitochondrial ETC. This complex is responsible for both electron transport and proton delivery, which are crucial for ATP generation and the production of reactive oxygen species (ROS), critical cell signaling molecules.
The researchers observed that impaired function of mitochondrial complex III led to a marked decrease in cellular respiration and the signaling molecules associated with ATP production. Furthermore, the loss of this complex in CD8+ T-cells resulted in diminished proliferation in response to viral infections. Notably, these cells exhibited rapid exhaustion upon acute antigen stimulation, a phenomenon previously linked only to chronic antigen exposure.
Additionally, the study found that deficient mitochondrial complex III function adversely affected the formation of CD8+ T-cell memory. This memory is essential for the immune system to respond effectively to subsequent infections. Chandel emphasized, “Every time you get a viral infection, you robustly respond to the virus and you clear it out, but you have a few memory T-cells that are sitting around that, if you get the same virus again, they proliferate quickly to protect you.”
In an innovative approach, the researchers introduced an alternative oxidase (AOX) protein derived from C. intestinalis into the mitochondrial complex III-deficient CD8+ T-cells. AOX can compensate for the loss of complex III without generating ROS. While the introduction of AOX effectively prevented T-cell exhaustion and aided in restoring cellular metabolism and proliferation, it did not restore memory formation. This outcome indicates that ROS generation is intrinsically linked to the ability of T-cells to form lasting memories.
Lead author Elizabeth Steinert, Ph.D., noted, “We saw memory precursor marker expression by those cells, showing that it’s not just an immediate death signal, but it’s a failure to form a terminal memory cell that can stick around.”
The comprehensive findings underscore the necessity of mitochondrial respiration for T-cell proliferation and memory formation, which could guide future therapeutic strategies targeting mitochondrial functions in these immune cells. Chandel concluded, “This tells you that mitochondrial metabolism prevents exhaustion, supports proliferation, and mitochondrial ROS is necessary to make memory. That really puts mitochondria at the center of T-cell biology, so maybe we should think about therapies that target mitochondria to rejuvenate them.”
For additional details, refer to the research by Elizabeth M. Steinert et al. titled “Mitochondrial respiration is necessary for CD8+ T cell proliferation and cell fate,” published in Nature Immunology in March 2025. DOI: 10.1038/s41590-025-02202-x.






