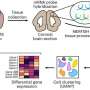
Research conducted by the SickKids Research Institute in Toronto and the University of Pennsylvania has unveiled significant findings regarding how maternal immune stress influences fetal brain development in mice. The study reveals that variations in immune-related genes occur by location and cell type within the developing mouse brain prior to birth, altering how fetal brain cells communicate.
The investigation, published in Nature Neuroscience in March 2026, highlights the effects of maternal immune activation and microbiome depletion on immune signaling patterns. Notably, the research identifies distinct differences in these patterns between male and female embryos. Immune molecules such as cytokines and chemokines play crucial roles in regulating synapse development, cellular communication, and the migration of neural precursor cells during brain development.
During fetal development, neurons are generated in one region, migrate to their designated locations, and form organized layers, particularly in the cortex. Proper alignment of these layers is vital, as different types of neurons occupy specific layers. Disruptions in the maternal immune system and microbiome have been associated with abnormal fetal neurodevelopment, affecting processes like neurogenesis and cellular migration.
Methodology and Findings
The research team employed a technique called multiplexed error-robust fluorescence in situ hybridization (MERFISH) to assess immune activity in embryonic mouse brains during mid and late gestation. This approach enabled them to analyze how maternal immune activation and microbiome depletion impacted immune signaling patterns. The study’s findings were supported by single-cell RNA sequencing data, which facilitated the identification of distinct cell populations and the analysis of differential gene expression.
The researchers examined 2.1 million cells across embryonic brains, with an average of 511 molecules detected per cell. They discovered that male embryos exposed to maternal immune activation or microbiome depletion exhibited a thicker deep-layer cortex and a reduced number of dividing cells in critical growth zones compared to those from saline-injected controls. Interestingly, female embryos did not display similar changes linked to microbiome depletion.
At eight to twelve weeks, adult offspring underwent social interaction and open-field tests. Results indicated that males from the maternal immune activation and microbiome depletion groups displayed altered social behaviors. Although the distance traveled and overall time spent interacting remained consistent across groups, males affected by maternal conditions spent less time in the center of the open field.
Sex-Specific Gene Activity Changes
The study also highlights significant differences in gene activity between male and female embryos following maternal immune activation. Male embryos showed decreased activity in genes associated with growth and division in early brain-building cells, such as Nes and Mki67. Conversely, males exhibited increased activity in genes linked to neural development, including Neurod1, Neurod2, and Neurog2. These patterns were not mirrored in female embryos.
Gene activity alterations due to maternal microbiome depletion were more consistent across sexes. Immune-related genes increased within precursor populations, indicating a complex interplay between maternal health and fetal brain development.
A key discovery from this research was the role of CXCL12, a chemokine crucial for cell-to-cell communication in the developing brain, and its receptor CXCR7. Both exhibited shifts following maternal immune activation and microbiome depletion, suggesting a potential common mechanism linked to neural progenitor abnormalities.
The study indicates that after maternal immune activation, the distances between various cell populations within the brain changed. For instance, precursor cells related to glial development became more distanced from developing inhibitory neurons, while early brain-building cells were farther from blood-vessel lining cells.
Overall, the research provides valuable insights into how maternal immune activation and microbiome depletion can lead to sex-specific changes in immune gene expression, signaling pathways, and cell arrangement within the embryonic brain. The findings emphasize the need for further investigations, including gain-of-function and loss-of-function experiments, to explore neuronal migration changes and behavioral outcomes in later life.
This research underscores the intricate relationship between maternal health and fetal brain development, paving the way for future studies aimed at understanding the underlying mechanisms involved. The implications of these findings could significantly impact approaches to maternal health and fetal neurodevelopment.