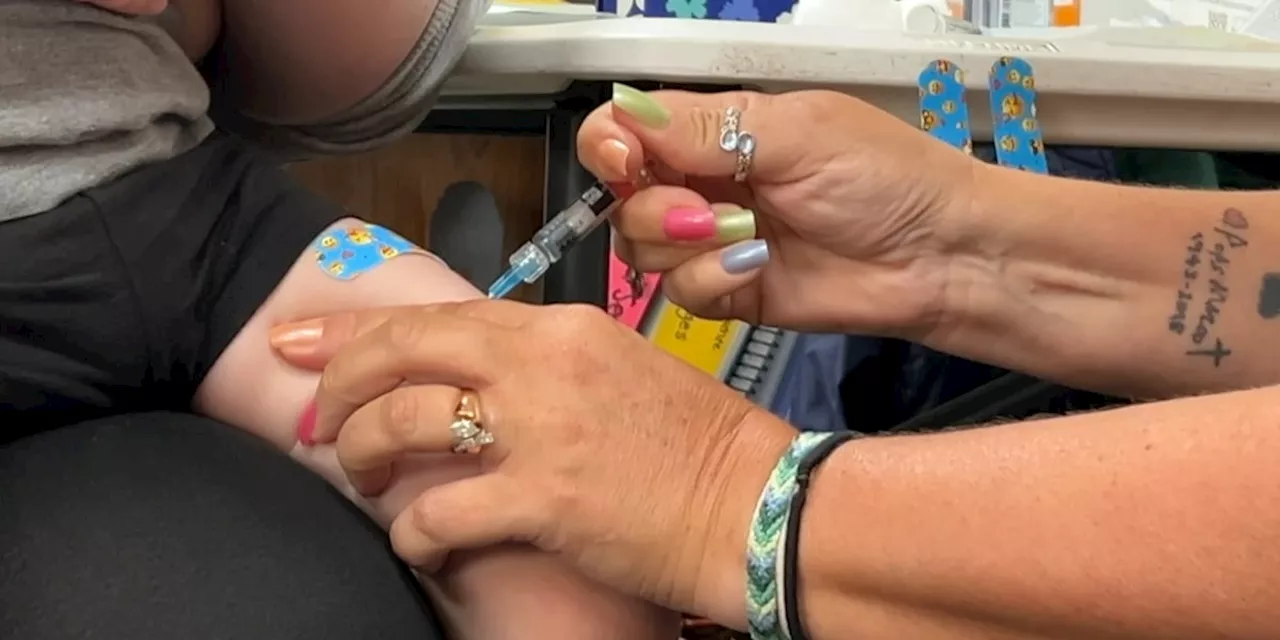
Grail’s innovative blood test has demonstrated improved accuracy in identifying true cancer cases, according to recent findings presented at the European Society for Medical Oncology (ESMO) Congress 2023. This advancement could significantly bolster the company’s position as it prepares to submit its application to the U.S. Food and Drug Administration (FDA) for regulatory approval.
Research findings indicate that Grail’s blood test is now more effective at distinguishing between positive results that indicate the presence of cancer and those that do not. In the study, the test achieved a notable increase in specificity, which is critical for patients and healthcare providers relying on accurate diagnostic tools.
Significance of the Findings
The enhanced performance of Grail’s blood test is particularly relevant as the global cancer burden continues to rise. With millions diagnosed each year, the demand for reliable early detection methods is crucial. The study showcased that the test correctly identified cancer in a higher percentage of true cases compared to previous iterations.
Dr. Eric Lefkofsky, CEO of Grail, emphasized the importance of this advancement during his presentation at the conference. He stated that “the ability to provide patients and doctors with more accurate information represents a significant leap in our fight against cancer.”
The upcoming FDA filing, expected in the coming weeks, aims to secure approval for the test, which is designed to detect multiple types of cancer through a simple blood draw. If approved, this could mark a transformative moment in cancer diagnostics, offering a less invasive option for early detection.
Market Impact and Future Prospects
Grail’s progress has attracted considerable interest from investors and healthcare professionals alike. The company is positioning itself as a leader in the liquid biopsy market, which is projected to grow to approximately $4.5 billion by 2026. The improved accuracy of its test could enhance its competitive edge, especially against other diagnostic companies also vying for market share.
As the FDA evaluates Grail’s application, the implications of these findings could extend beyond the company itself. An increase in accurate cancer detection could lead to earlier interventions, which are often associated with improved patient outcomes. This is a significant consideration for healthcare systems worldwide, where timely diagnosis can greatly influence treatment success rates.
In summary, Grail’s blood test has shown promising improvements in its ability to accurately identify cancer cases. With the impending FDA submission, the company is on the brink of potentially redefining cancer diagnostics, paving the way for better patient care and outcomes in the future.