Eisai Co., Ltd. has presented significant findings regarding its novel orexin receptor agonist, E2086, during the World Sleep 2025 congress in Singapore. On September 8, 2025, the company revealed results from a Phase Ib clinical trial demonstrating E2086’s potential to enhance wakefulness in individuals living with narcolepsy type 1 (NT1).
Clinical Trial Overview
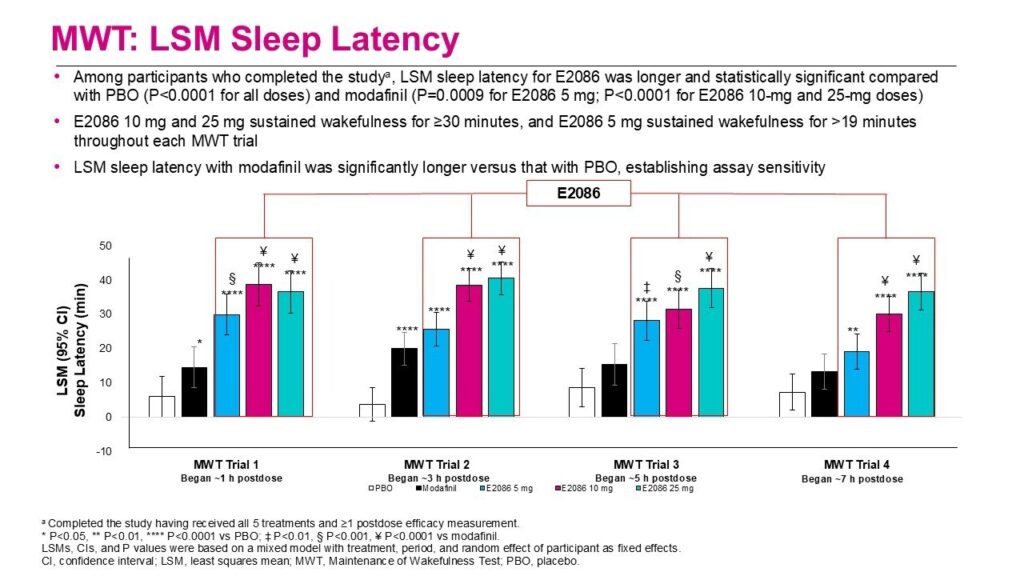
The randomized, double-blind clinical trial (NCT06462404) involved participants from the United States and Canada, focusing on the efficacy, safety, and tolerability of E2086. A total of 21 patients diagnosed with NT1 were enrolled, with 19 completing the trial. Each participant received one of five treatments: E2086 at doses of 5 mg, 10 mg, or 25 mg, a placebo, or modafinil, shortly after waking. The study evaluated efficacy through the Maintenance of Wakefulness Test (MWT), an objective assessment of excessive daytime sleepiness (EDS), alongside the subjective Karolinska Sleepiness Scale (KSS).
Results indicated that all doses of E2086 significantly improved sleep latencies compared to placebo (P<0.0001) and exhibited better outcomes than modafinil for the 5 mg dose (P=0.0009) and the higher doses (P<0.0001). The KSS results further supported these findings, showing that all E2086 doses provided greater alertness versus placebo (P<0.0001), with the 10 mg and 25 mg doses outperforming modafinil (P<0.0001).
Safety Profile and Expert Commentary
Treatment-emergent adverse events (TEAEs) were monitored throughout the study. Among the 21 patients who received the study medication, common TEAEs included increased urinary frequency, nausea, dizziness, and urinary urgency. Importantly, no severe TEAEs were reported, and all incidents were categorized as mild to moderate in severity.
Dr. Katsutoshi Ido, Chief Scientific Officer at Eisai, commented on the trial’s findings, stating, “The data presented at World Sleep 2025 highlights E2086’s potential to enhance daytime wakefulness in individuals with narcolepsy type 1. This condition presents a significant healthcare challenge, characterized by excessive daytime sleepiness and related symptoms that severely impact quality of life. The study’s efficacy and safety results justify further exploration in the narcolepsy population.”
E2086 represents Eisai’s continued commitment to addressing sleep disorders. The drug functions by activating orexinergic neurons, which are crucial for regulating wakefulness. This approach builds on the company’s previous work with DAYVIGO (generic name: lemborexant), an orexin receptor antagonist already available in over 25 countries.
As Eisai progresses with the development of E2086, the company aims to provide new treatment options for those impacted by narcolepsy, a chronic condition that significantly burdens patients due to its effects on fatigue, cognition, and persistent symptoms despite existing treatments.
With these promising results, Eisai is poised to make strides in the management of narcolepsy, potentially transforming the lives of countless individuals affected by this challenging disorder.






