Cardiff Oncology, Inc. has announced significant findings from its ongoing clinical trial evaluating the effectiveness of onvansertib in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). The trial, named CRDF-004, revealed that the 30mg dose of onvansertib achieved a confirmed objective response rate (ORR) of 49%, compared to 30% in the control arm, based on data collected up to July 8, 2025. This data was assessed through a blinded, independent central review of tumor scans in a patient population of 110.
Roger Sidhu, MD, Chief Medical Officer of Cardiff Oncology, expressed optimism about the trial’s outcomes, highlighting a notable 19% improvement in confirmed ORR. The data also indicated a trend favoring the 30mg dose of onvansertib in terms of early progression-free survival (PFS) compared to the control group. “The totality of the data we are releasing today strengthens the initial findings from our December 2024 data release in a significantly larger patient population,” Sidhu stated.
Trial Design and Efficacy Data
The CRDF-004 trial enrolled patients with documented KRAS or NRAS mutations and administered onvansertib alongside standard-of-care (SoC) treatments, including FOLFIRI with bevacizumab or FOLFOX with bevacizumab. Participants were randomized into six arms, comparing different dosages of onvansertib (20mg and 30mg) against SoC alone. The primary endpoint of the trial is the ORR, while secondary endpoints include PFS, duration of response (DOR), and safety.
Early results show that both the 20mg and 30mg doses of onvansertib led to an early separation of PFS curves compared to the control group, though the median PFS has not yet been reached. Notably, the analysis demonstrated a dose-dependent effect, with the 30mg group exhibiting improved outcomes.
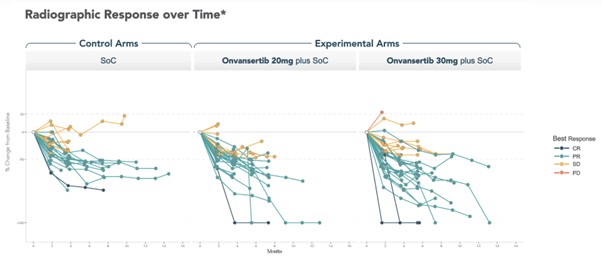
Spider plots illustrating changes in tumor size over time revealed that patients receiving the higher dose of onvansertib experienced greater tumor shrinkage compared to both the control arm and the 20mg dose arm. The confirmed ORR, according to RECIST v1.1 criteria, includes patients achieving either a complete response or partial response, assessed via repeat imaging.
Safety Profile and Next Steps
Safety assessments were conducted on 104 patients involved in the trial. Onvansertib, when combined with chemotherapy and bevacizumab, was generally well tolerated, with no major or unexpected toxicities reported. Incidents of Grade 3 or higher adverse events were infrequent, with neutropenia cited as the most common treatment-emergent adverse event.
Mark Erlander, Chief Executive Officer of Cardiff Oncology, reaffirmed the company’s commitment to advancing onvansertib’s development. “We are highly encouraged by the strength of our data which achieves the key objectives we set for the trial, and positions us to engage in discussions with the FDA as we advance toward our registrational CRDF-005 trial,” Erlander noted. He expressed optimism that onvansertib could redefine first-line treatment for RAS-mutated mCRC, with updates on the program expected by the first quarter of 2026.
Cardiff Oncology plans to host a conference call and live webcast today at 4:30 p.m. ET / 1:30 p.m. PT, where further details regarding the trial and the company’s future plans will be discussed.
For more information about Cardiff Oncology and its clinical programs, visit the company’s official website.







