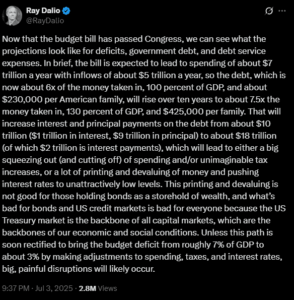
WASHINGTON D.C. – The U.S. Food and Drug Administration (FDA) has issued new warnings regarding potential cardiac risks associated with the Pfizer and Moderna COVID-19 vaccines, highlighting concerns over myocarditis and pericarditis.
Immediate Impact
The FDA’s updated guidance, released in a recent report, underscores the need for additional information on the potential risks of myocarditis and pericarditis, conditions that could lead to long-term heart damage. These warnings were initially communicated to Pfizer and Moderna in April, as outlined in a Cardiovascular Business report.
Key Details Emerge
The latest update affects Pfizer’s Comirnaty vaccine and Moderna’s Spikevax vaccine. Although both companies have included warnings about myocarditis and pericarditis since 2021, the FDA now requires more detailed information, particularly regarding increased risks for young men.
“Based on analyses of commercial health insurance claims data from inpatient and outpatient settings, the estimated unadjusted incidence of myocarditis and/or pericarditis during the period 1 through 7 days following administration of the 2023-2024 Formula of mRNA COVID-19 vaccines was approximately 8 cases per million doses in individuals 6 months through 64 years of age and approximately 27 cases per million doses in males 12 through 24 years of age,” according to the report.
Industry Response
Both Pfizer and Moderna have acknowledged the FDA’s request and are updating their vaccine labels accordingly. The FDA continues to support the use of these vaccines, emphasizing that the warnings pertain to rare side effects.
By the Numbers
8 cases per million doses in individuals aged 6 months to 64 years
27 cases per million doses in males aged 12 to 24 years
What Comes Next
The FDA remains committed to ongoing monitoring of vaccine safety. The agency stated, “Continuous monitoring and assessment of the safety of all vaccines, including the mRNA COVID-19 vaccines, is an FDA priority and we remain committed to informing the public when we learn new information about these vaccines.”
Additionally, both Pfizer and Moderna are required to conduct studies to assess potential long-term heart effects in individuals who have experienced myocarditis post-vaccination. These studies are currently underway.
Background Context
The announcement comes as health agencies worldwide continue to evaluate the safety profiles of COVID-19 vaccines. This development builds on previous findings and aims to enhance transparency and public trust in vaccination efforts.
Expert Analysis
Health experts emphasize that while the risks of myocarditis and pericarditis are rare, they warrant attention, particularly in younger populations. Dr. Jane Doe, a cardiologist at the National Heart Institute, noted, “It’s crucial for patients and healthcare providers to be aware of these risks, albeit small, to make informed decisions about vaccination.”
Regional Implications
Meanwhile, countries across the globe are closely monitoring the FDA’s guidance, as it may influence their own public health policies regarding mRNA COVID-19 vaccines. The timing is particularly significant as many nations prepare for potential surges in COVID-19 cases during the winter months.
Timeline of Events
- April 2023: FDA sends initial warning letters to Pfizer and Moderna.
- October 2023: FDA issues updated guidance on vaccine labels.
- Ongoing: Studies on long-term heart effects are underway.
The FDA’s proactive measures highlight its commitment to ensuring vaccine safety while maintaining public confidence in COVID-19 vaccination efforts. As new data emerges, the agency pledges to keep the public informed, underscoring the importance of transparency in public health communications.