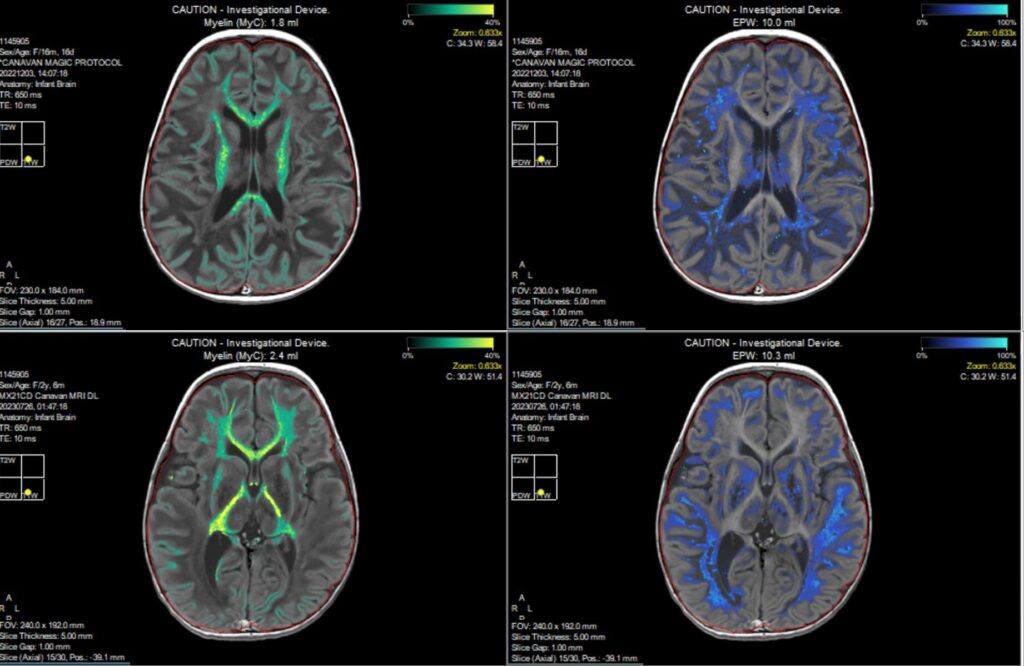
SyMRI image of Canavan patient before and 12 months post treatment shows improvement in myelin volume and brain water reduction.
Myrtelle Inc. has announced significant interim results from its Phase 1/2 clinical trial of the investigational gene therapy rAAV-Olig001-ASPA (MYR-101) for Canavan disease, published in the prestigious journal Nature Medicine on September 16, 2025. This innovative therapy aims to address the critical enzymatic deficiency caused by mutations in the ASPA gene, offering new hope to children affected by this debilitating genetic disorder.
The study highlights that the targeted oligodendrocyte gene therapy leads to decreased levels of N-acetylaspartate (NAA) in cerebrospinal fluid (CSF) and increased brain myelin volume, resulting in promising functional improvements in young patients. “The reductions in CSF NAA levels, gains in myelin volume, and functional improvements in treated patients mark a compelling step forward for patients who currently have no approved treatment options,” stated Adrian Stecyk, Chief Executive Officer of Myrtelle. He emphasized that the publication underscores the potential of rAAV-Olig001-ASPA as a disease-modifying therapy for Canavan disease.
Details of the Clinical Trial
Myrtelle’s First-in-Human (FIH) trial involved eight children diagnosed with typical Canavan disease, monitored for up to two years post-treatment. The therapy utilizes a proprietary recombinant adeno-associated virus vector to deliver a functional ASPA gene directly to oligodendrocytes. In Canavan disease, these cells are unable to produce the enzyme aspartoacylase, which is crucial for myelin production and proper metabolism of NAA.
Key findings from the trial indicate that MYR-101 therapy was well-tolerated, with no serious adverse events linked to the gene therapy itself. Notably, significant reductions in NAA levels in CSF provide early evidence of its therapeutic effect. Synthetic MRI (SyMRI) results showed substantial increases in brain myelin volume, suggesting new myelination. Participants demonstrated developmental improvements compared to historical controls, assessed by the Mullen Scales of Early Learning (MSEL). All participants improved in at least two MSEL domains, with many showing progress in three or more, reflecting broad functional gains.
The study supports the potential of MYR-101 as a disease-modifying therapy, with ongoing follow-up assessing long-term outcomes and durability of response.
Regulatory Progress and Designations
Myrtelle’s rAAV-Olig001-ASPA has recently been selected by the U.S. Food and Drug Administration (FDA) for inclusion in the Support for Clinical Trials Advancing Rare Disease Therapeutics (START) pilot program. This initiative, of which MYR-101 is one of only four CBER-regulated gene therapies to receive this recognition, provides enhanced regulatory support to expedite the development of promising therapies for rare diseases.
Additionally, the therapy holds several regulatory designations, including Regenerative Medicine Advanced Therapy (RMAT), Orphan Drug, Rare Pediatric Disease, and Fast Track designations from the FDA. It has also received Orphan Drug Designation and Advanced Therapy Medicinal Product (ATMP) classification from the European Medicines Agency (EMA), along with the Innovative Licensing and Access Pathway (ILAP) designation from the UK Medicines and Healthcare Products Regulatory Agency (MHRA).
Understanding Canavan Disease
Canavan disease is a severe genetic brain disorder caused by mutations in the ASPA gene that disrupt normal enzyme production in oligodendrocytes. The absence of aspartoacylase leads to impaired brain function, affecting myelin production and resulting in significant developmental challenges. Symptoms often appear in infancy, with affected children displaying poor head control, an abnormally large head size, and delays in motor skills. As the disease progresses, complications such as seizures, spasticity, and muscle deterioration typically develop, ultimately leading to life-threatening issues by around ten years of age. Currently, there are no cures for Canavan disease, and available treatments focus solely on providing palliative care.
Myrtelle’s innovative approach represents a significant step forward in the search for effective treatments for neurodegenerative diseases. For additional information regarding the clinical trial for Canavan disease, please visit https://clinicaltrials.gov/ under the identifier NCT04833907 or contact Myrtelle directly at [email protected].
As Myrtelle continues its research and development efforts, the results from this clinical trial may pave the way for a transformative therapy that addresses a critical unmet need in the treatment of Canavan disease.