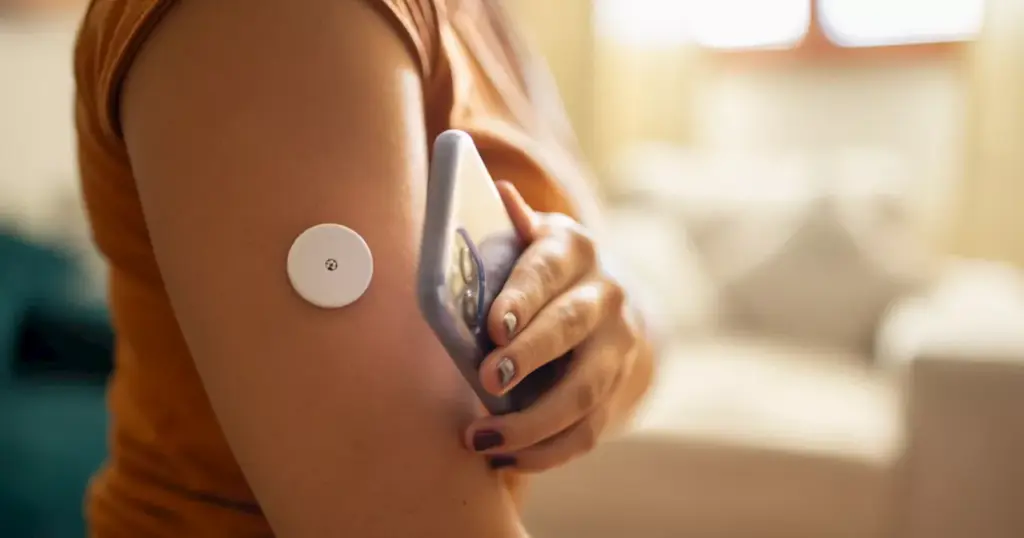
The U.S. Food and Drug Administration (FDA) has issued a significant alert regarding two glucose monitor sensors produced by Abbott Diabetes Care, which have been linked to multiple fatalities. The FDA’s announcement highlights a “potentially high-risk issue” stemming from malfunctioning devices that provide incorrect low glucose readings, potentially leading to severe health consequences.
As of November 14, 2023, the FDA reported that Abbott had documented 736 serious injuries and seven deaths attributed to this malfunction. The affected products, the FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors, are designed to assist individuals aged four and older in managing their glucose levels. According to Abbott, these sensors have a manufacturing issue that significantly impacts their accuracy.
In a press release dated November 24, Abbott confirmed that over 3 million FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors were affected. The company emphasized the critical risks associated with prolonged exposure to incorrect low glucose readings. If not addressed, these inaccuracies could lead to misguided treatment decisions, such as excessive carbohydrate consumption or delaying insulin doses, which may ultimately result in serious health risks, including potential injury or death.
Abbott has indicated that it has “identified and resolved the cause of the issue” affecting the sensors. Despite this, the company continues to manufacture devices to meet replacement and new order demands. Customers are advised to utilize a blood glucose meter or the built-in meter in the FreeStyle Libre 3 reader when sensor readings do not align with symptoms or expectations.
In a statement to customers, Abbott has outlined the necessary actions for those who may have encountered errors with their devices. The company has proactively reached out to customers to provide guidance and support. Individuals can visit www.FreeStyleCheck.com to determine if their sensors are affected and to request replacements at no cost.
Abbott has urged customers to cease usage of the affected sensors and to dispose of them properly. The specific models impacted include:
– **FreeStyle Libre 3 Sensor**
– Model Numbers: 72081-01, 72080-01
– Unique Device Identifiers: 00357599818005, 00357599819002
– **FreeStyle Libre 3 Plus Sensor**
– Model Numbers: 78768-01, 78769-01
– Unique Device Identifiers: 00357599844011, 00357599843014
Customers can locate the serial number on their devices via the accompanying app or reader, or on the label at the bottom of the sensor or carton. A comprehensive list of all affected lot numbers is available through Abbott.
The FDA encourages anyone who has experienced adverse reactions or issues with the sensors to contact Abbott Diabetes Care directly at 1-833-815-4273. Additionally, both healthcare professionals and consumers are advised to report any reactions or problems to the FDA’s MedWatch program, which is dedicated to safety information and adverse event reporting.
As the situation develops, it remains crucial for users of the FreeStyle Libre sensors to stay informed and ensure their health and safety.






