On November 14, 1985, a team of chemists at Rice University in Houston, Texas, announced the discovery of a new molecule that would revolutionize the field of chemistry. Known as “buckyballs,” these molecules of carbon possess a unique structure and remarkable properties, marking a significant milestone in molecular science.
The Journey to Discovery
The journey leading to the discovery of buckyballs began in the 1970s, with Harry Kroto, a chemist at the University of Sussex in the United Kingdom. Kroto was investigating the presence of organic molecules in interstellar space, particularly in the vast clouds that exist between stars. He noted, during his Nobel Prize speech, that radio and light data suggested a higher abundance of long carbon chains than was predicted by the astrophysical theories of the time.
This observation raised questions about the sources of these molecules, leading scientists to theorize that cooling red giant stars could be contributing to the formation of these carbon chains. In pursuit of deeper understanding, Kroto visited Rice University in September 1985, where he collaborated with chemists Richard Smalley and Robert Curl.
Uncovering Buckyballs
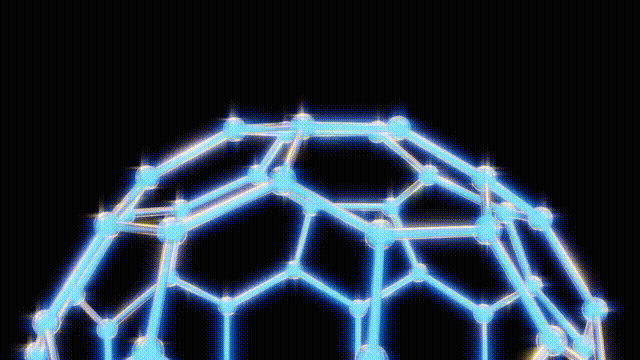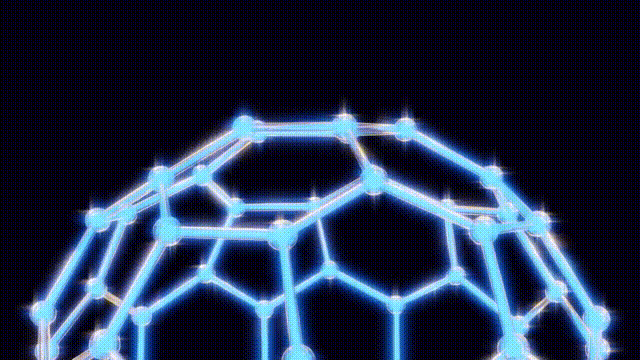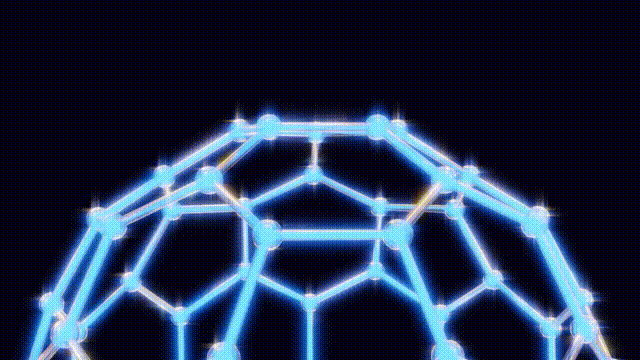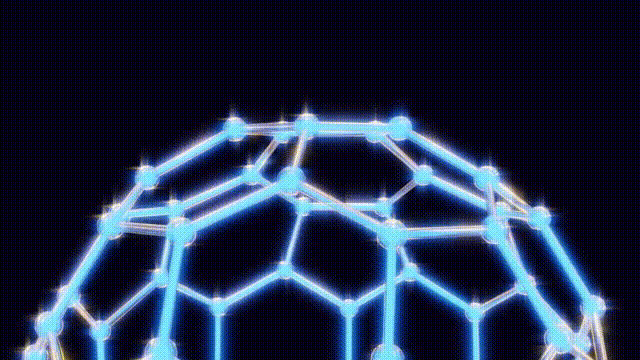
During a ten-day research period, Kroto, Smalley, and Curl utilized a unique apparatus designed by Smalley, which involved vaporizing carbon atoms with a laser and trapping them in a helium environment for analysis. By replacing the metal disk typically used with one made of graphite, they aimed to replicate conditions similar to those found in red giant stars. This innovative approach led to the unexpected detection of a new form of carbon that comprised 60 carbon atoms—later identified as C60.
Initially, the discovery was met with uncertainty as the team tried to comprehend the structure of this newfound molecule. They engaged in creative modeling, using toothpicks, jellybeans, and paper cutouts to visualize the arrangement of atoms. The breakthrough came when Kroto recalled the work of futurist Buckminster Fuller, known for his geodesic dome designs. This connection inspired the naming of the compound as “buckminsterfullerene,” which reflects its geometric structure.
The groundbreaking research was published in the journal Nature, further solidifying the significance of the discovery. The term “buckyballs” quickly gained traction, capturing the imagination of the scientific community and the public alike.
Impact and Future Research
In subsequent years, the research team explored the properties of fullerenes, leading to the recognition of their potential applications. In 1996, Kroto, Smalley, and Curl were awarded the Nobel Prize in Chemistry for their pioneering work. The implications of their discovery extend beyond academic interest; fullerenes and their derivatives, such as carbon nanotubes, exhibit exceptional strength and conductivity.
These materials have found applications in various fields, including atomic force microscopy, energy storage solutions like batteries, and biosensors. Although there have been numerous proposals for using buckyballs in advanced technologies such as quantum computing and drug delivery systems, they have yet to achieve widespread commercial application.
The discovery of buckyballs stands as a testament to the ingenuity of scientific exploration. As researchers continue to investigate the properties and potential uses of fullerenes, the legacy of Kroto, Smalley, and Curl remains a pivotal chapter in the history of chemistry.