A research team from the School of Engineering at The Hong Kong University of Science and Technology (HKUST) has made a significant advancement in brain imaging technology. They have developed the world’s first method to capture high-resolution images of the brains of awake mice in a nearly noninvasive manner. This breakthrough eliminates the need for anesthesia, allowing researchers to study brain tissue in its fully functional state, thereby paving the way for deeper insights into human brain functions in both healthy and diseased conditions.
Published in Nature Communications on March 15, 2025, the study titled “Rapid adaptive optics enabling near-noninvasive high-resolution brain imaging in awake behaving mice” outlines the implications of this innovation for neuroscience research. Mice often serve as model organisms for investigating treatments for neurological disorders such as Alzheimer’s disease, Huntington’s disease, and epilepsy, as well as therapies for various cancers and vaccine efficacy. Traditionally, anesthesia has been used in such studies, but it significantly alters physiological processes, leading to unreliable results.
Understanding the human brain’s complexities has long posed challenges for scientists. Current imaging methods, including magnetic resonance imaging (MRI), electroencephalography (EEG), computed tomography (CT), and positron emission tomography (PET), often fall short in revealing the intricate details of brain activity. Anesthesia can disrupt blood circulation, glial cell morphology, and neuronal activity, further complicating research.
The newly developed technology, known as Multiplexing Digital Focus Sensing and Shaping (MD-FSS), was spearheaded by Prof. QU Jianan, a professor in the Department of Electronic and Computer Engineering at HKUST. This advancement builds on Prof. Qu’s previous work, ALPHA-FSS, which achieved subcellular resolution using three-photon microscopy. Despite its precision, ALPHA-FSS struggled with speed, making it challenging to capture high-quality images of awake animals where natural movements often resulted in blurred images.
The MD-FSS technology addresses these limitations by drastically accelerating the measurement of the point spread function (PSF)—the three-dimensional image of a point-like object under the microscope. By directing multiple spatially separated weak laser beams alongside a strong primary beam, the system generates nonlinear interference within the brain. Each beam carries distinct spatial information encoded at unique frequencies. The system can measure PSF in less than 0.1 seconds, achieving speeds over ten times faster than previous methods.
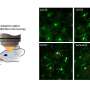
This enhanced capability allows researchers to observe dynamic brain activity with sharp, precise images. The resolution provided by multiphoton microscopy is significantly higher than that of conventional methods, enabling the visualization of individual neurons, immune cells, and the smallest capillary structures and their functions.
Adaptive Optics Three-photon Microscopy, developed by integrating MD-FSS with multiphoton microscopy, has demonstrated the ability to track functional changes in brain immune cells, measure blood flow in tiny cerebral vessels, and monitor neuronal activity during cognitive and sensory processing. Prof. Qu noted, “Such detailed, near-noninvasive, and real-time observations in awake animals were previously impossible. With the rapid aberration-correction capability of this novel adaptive optics technology, high-quality imaging is now achievable without injuring the subject’s brain.”
MD-FSS is designed for scalability, with the current system employing eight beams for PSF measurement. It can be expanded to dozens or even hundreds of beams, facilitating faster and broader imaging as light-control technologies evolve. Prof. Qu emphasized that this development represents much more than an incremental improvement; it offers a versatile platform for faster imaging, larger brain region studies, and integration with functional assays.
This innovation empowers neuroscientists to investigate rapid brain events, complex network interactions, and disease progression in ways previously deemed unattainable, potentially leading to transformative discoveries in learning, memory, mental health, and neurological disorders.
For further details, refer to the study conducted by Zhentao She et al, published in Nature Communications.