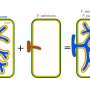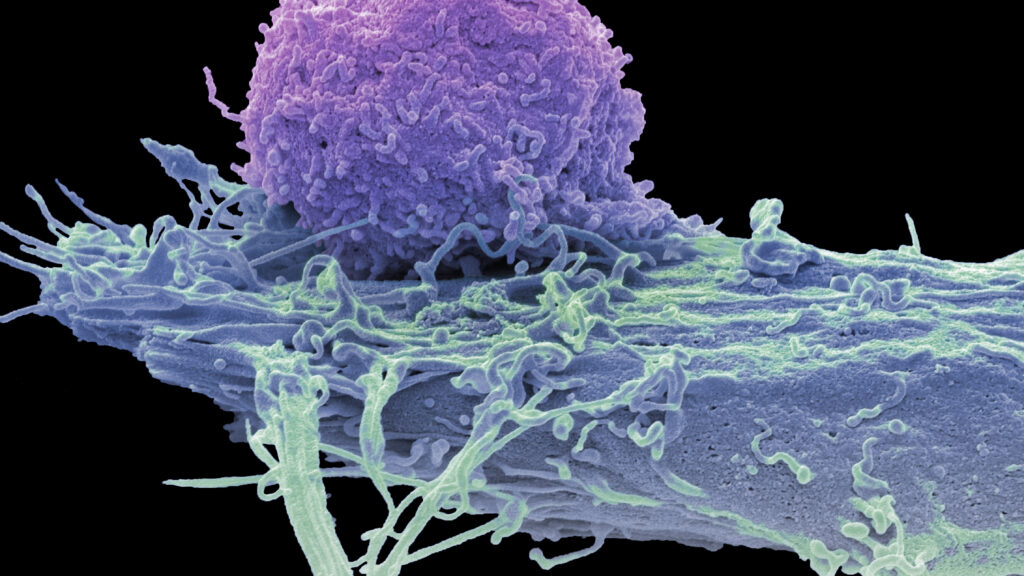
Experimental CAR-T therapies developed by Cabaletta Bio and Bristol Myers Squibb have demonstrated the ability to induce complete remissions in patients with a severe inflammatory muscle disease, according to findings from dual clinical trials presented recently. These preliminary results contribute to a growing body of evidence suggesting that personalized cell therapies, which have shown success in treating blood cancers, may also offer curative options for individuals suffering from serious autoimmune disorders.
The implications of this research are significant. Patients with severe autoimmune diseases frequently face the burden of taking multiple medications daily, which can lead to high health and financial costs. Steven Nichtberger, CEO of Cabaletta, emphasized the transformative potential of a single CAR-T treatment, stating, “These are patients who take three to five medicines every day, every week, every month, at great cost both healthwise and financially. With a one-time CAR-T treatment, we’re showing we can eliminate all of those drugs, giving them the opportunity to no longer be patients. We are freeing them from their disease.”
Clinical Trials and Results
The dual clinical trials involved a group of patients diagnosed with severe inflammatory muscle disease, a condition characterized by debilitating inflammation and muscle weakness. The studies aimed to assess the effectiveness and safety of the CAR-T therapies administered to these patients.
While the results are still in the preliminary phase, the response observed in the trial participants has garnered attention within the medical community. Initial data indicate a high rate of remission, suggesting that these therapies could significantly alter the treatment landscape for autoimmune conditions.
As the research progresses, both Cabaletta Bio and Bristol Myers Squibb are keen to further investigate the long-term efficacy and safety of these therapies. The potential for CAR-T treatments to replace conventional medication regimens could mark a pivotal shift in how autoimmune disorders are managed.
Future Implications
The ongoing exploration of CAR-T therapies reflects a broader trend towards personalized medicine, where treatments are tailored to the individual characteristics of patients. This approach not only holds promise for improved health outcomes but also aims to reduce the overall burden of chronic disease management.
The findings from these trials could pave the way for additional research into similar therapies, potentially expanding the range of conditions that can be treated effectively with CAR-T technology. As the medical community awaits further data, the hope is that these innovative therapies will bring relief to countless patients grappling with the challenges of autoimmune disorders.
In conclusion, the preliminary findings from the dual clinical trials conducted by Cabaletta Bio and Bristol Myers Squibb signify an important advancement in the treatment of severe inflammatory muscle disease. With ongoing research, CAR-T therapies may soon offer a viable alternative for patients currently reliant on multiple medications, transforming lives and enhancing the quality of care in autoimmune disease management.